Risk of Galvanic Corrosion
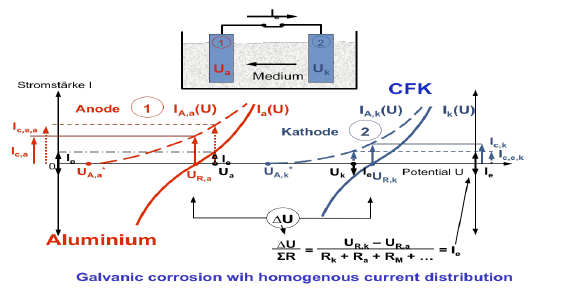
A risk of galvanic corrosion respectively contact corrosion is given when constructions are built with mixed materials. For example in lightweight constructions, Aluminium and CFRP (Carbon fibre reinforced plastic) are often used together. In case of mixed material construction a galvanic cell can establish when the conductive materials are in contact, and when a water film is present on the surface. E.g. automobiles and aeroplanes are exposed to the atmosphere and therefore, water can cover the outer material surface by rainwater, precipitation of moisture or fog. Inside of an aeroplane or of an automobile, the condensation of humidity causes a thin film of water on the surface. A formation of a water film cannot be avoided, particular in aeroplanes since the huge temperature changes during the take off and landing could lead to condensed water on the surface. The water can form a conductive pathway between the different materials. In the water, the electrochemical reactions occur at the water/metal and water/CFRP interface with metal dissolution and O2 reduction.
In case of a galvanic cell, the less noble material will corrode. Aluminium is less noble than CFRP. Aluminium is then the anode and CFPR the cathode.